This site is intended for Healthcare Professionals only. if you are a member of the general public, click here
ZEPOSIA was generally well tolerated in patients with moderate-to-severe
UC in the TRUE NORTH study4
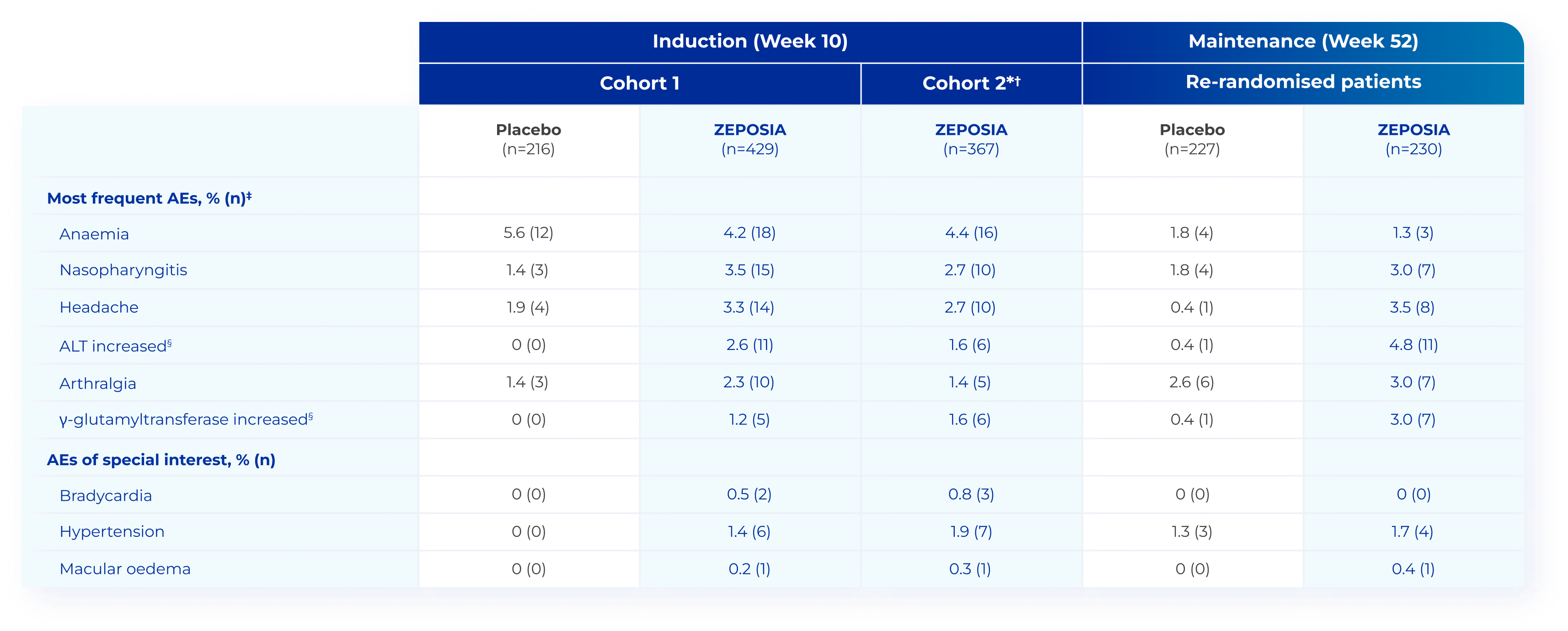
Adapted from Sandborn WJ et al. 2021.4
 Pinch & zoom to explore
Pinch & zoom to explore
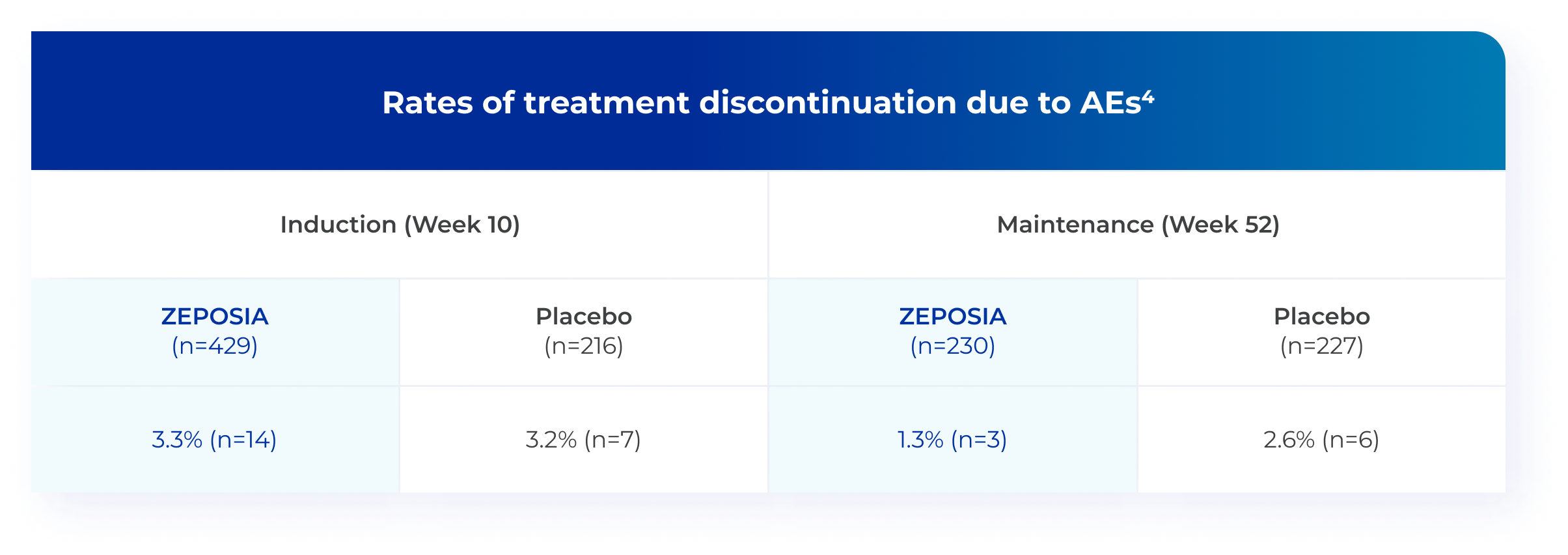
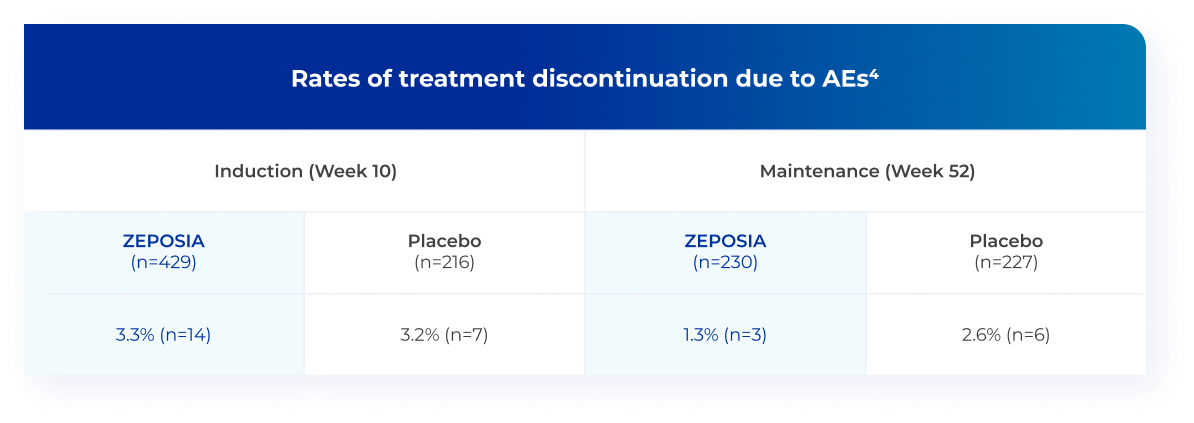
ZEPOSIA was generally well tolerated, with low and comparable
discontinuation rates vs placebo in TRUE NORTH4
Please refer to the ZEPOSIA Summary of Product Characteristics for full safety information
*Cohort 2 was included to increase the number of patients with a response who would be available for randomisation in the maintenance phase of the trial.4
†One death occurred in Cohort 2 in a patient with a history of ischaemic cardiomyopathy and prolonged tobacco use, in whom influenza and acute respiratory distress syndrome developed.4
‡The most frequent events were defined as those that occurred in at least 3% of the patients who received ZEPOSIA during the induction or maintenance period.4
§Laboratory values were flagged by the central laboratory if they fell outside the standard reference range. The investigator decided whether the laboratory value qualified as an AE.4
AE, adverse event; ALT, alanine aminotransferase; CV, cardiovascular; MACE, major adverse cardiac event; MI, myocardial infarction; UC, ulcerative colitis.
In TRUE NORTH, cardiovascular serious AEs in the ZEPOSIA arm
were uncommon compared to placebo7
- Induction phase, ZEPOSIA (n=429) vs. placebo (n=216): Cardiac disorders 1.4% vs 0.9%; vascular disorders 2.3% vs. 0.5%7
- Maintenance phase, ZEPOSIA-placebo (n=227) vs. ZEPOSIA-ZEPOSIA (n=230) vs. placebo (n=69): Cardiac disorders 0% vs. 1.3% vs 2.9%; vascular disorders 1.8% vs. 2.6% vs. 1.4%7

No cases of myocardial infarction, pulmonary embolism, or deep vein thrombosis associated with ZEPOSIA in the induction phase7

No cases of bradycardia, stroke, myocardial infarction, pulmonary embolism, or deep vein thrombosis associated with continuous ZEPOSIA use in the maintenance phase7

No deaths related to cardiovascular events7
Prior to treatment initiation with ZEPOSIA, an ECG in all patients should be obtained to determine whether any pre-existing cardiac abnormalities are present. In patients with certain pre-existing conditions, first-dose monitoring is recommended.1
AE, adverse event; ECG, electrocardiogram.
References
- ZEPOSIA (ozanimod) Summary of Product Characteristics, 2023.
- Sandborn WJ et al. N Engl J Med. 2016;374(18):1754–1762.
- D’Haens G et al. Oral presentation at: European Crohn’s and Colitis Organisation; Stockholm, Sweden; 21–24 February, 2024. Poster: P1009.
- Sandborn WJ et al. N Engl J Med. 2021;385(14):1280–1291.
- Cree BA et al. Mult Scler. 2022;28(12):1944–1962.
- Selmaj KW et al. Oral presentation at: European Academy of Neurology; 2023. Presentation: EPR-299.
- Long M et al. Long-term cardiac safety of ozanimod in phase 3 clinical program of ulcerative colitis and relapsing multiple sclerosis. Presented at: Digestive Disease Week (DDW); San Diego, CA, and Virtual, May 21–24, 2022. Presentation 15.