This site is intended for Healthcare Professionals only. if you are a member of the general public, click here
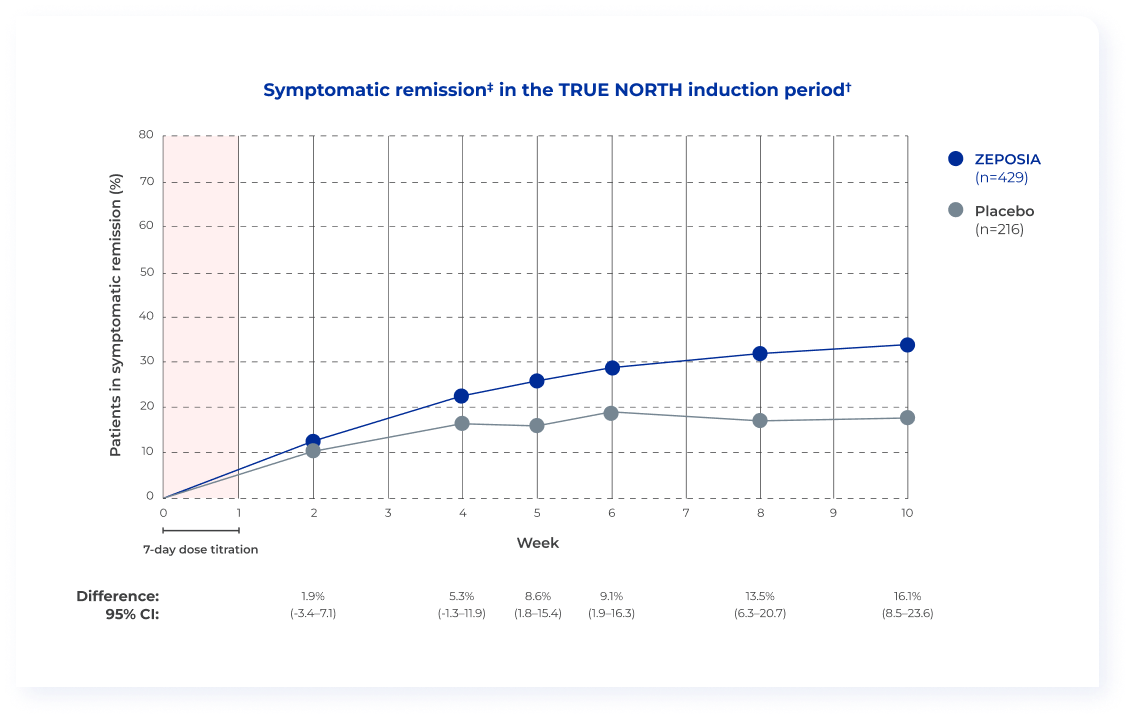
Patients on ZEPOSIA achieved rapid symptomatic relief one week after
completing the 7-day dose titration (post hoc analysis)1
Symptomatic response* in the
TRUE NORTH induction period†
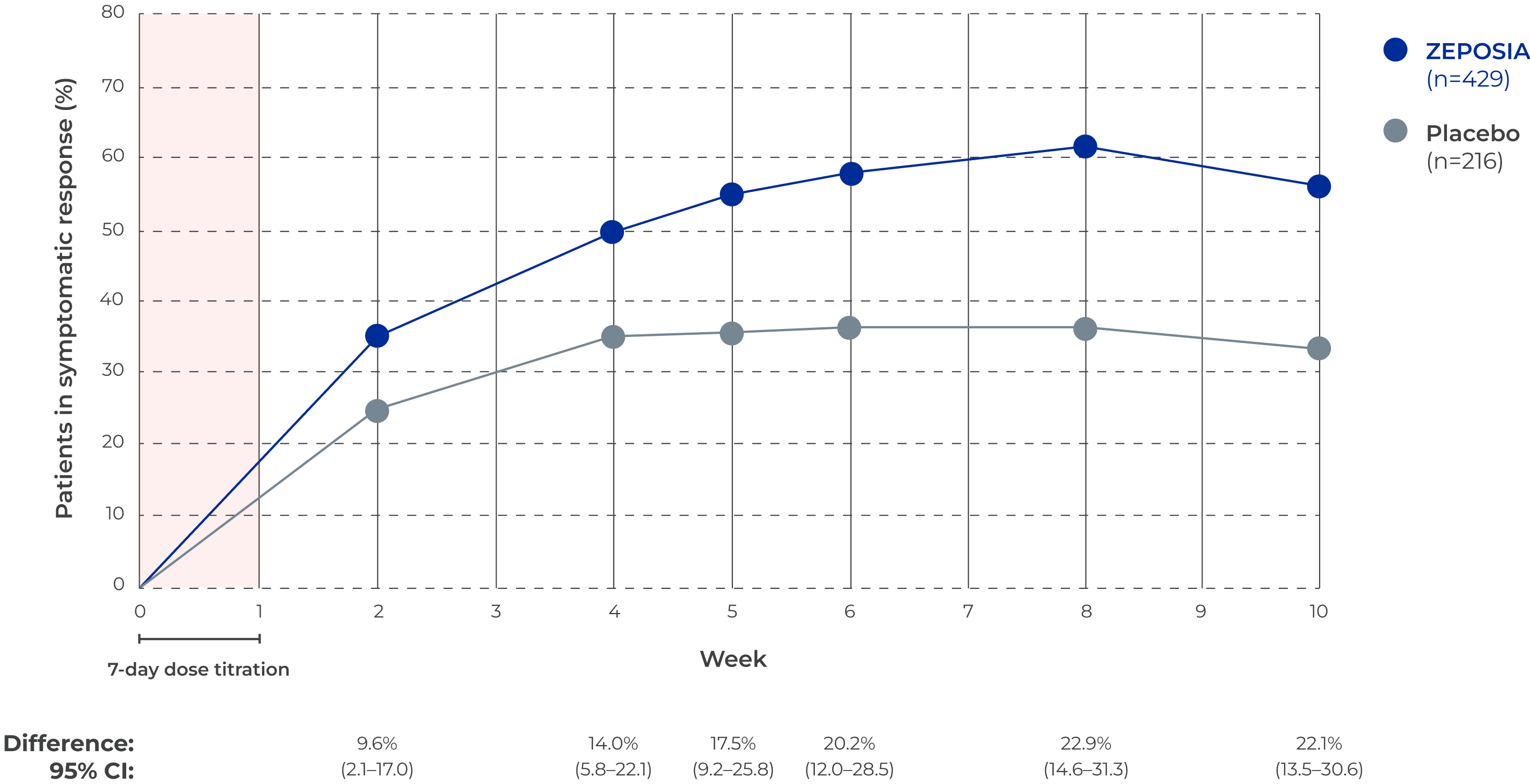

Adapted from Siegmund B et al. 2022.1
 Pinch & zoom to explore
Pinch & zoom to explore
Reference
- Siegmund B et al. Poster presented at: European Crohn’s and Colitis Organisation, Virtual; 16–19 February, 2022. Poster: DOP43.